A Systematic Review of Medical Equipment Reliability Assessment in Improving the Quality of Healthcare Services

Introduction
The growing sophistication of medical equipment has significantly improved the individual and society’s health (1). The advancement has improved survivability in the face of disease or injury and greatly enhanced patients’ life quality through an improved diagnosis and therapeutic results. Managing assets and facilities is one of the significant features in ensuring the continuity of primary and support business activities in healthcare services (2). The delivery of healthcare services to the communities are significantly affected without effective management implementation (3–5). Medical equipment is a crucial asset that substantially contributes to the effectiveness and healthcare services quality enhancement (6, 7). As the medical equipment aids various services in the healthcare sector, the management representative, such as clinical engineers, must monitor and upkeep the assets by performing several maintenances works throughout the equipment life cycle (8, 9).
Maintenance management of medical equipment is crucial to ensure that a machine operates in accordance with manufacturer specifications and guarantees the patients and users safety (10). Failure of medical equipment may affect the healthcare services effectiveness and cause severe injury to the patients and harm the environment (11). Bahreini et al. (12) summarised that the affecting factors are management, resources, information bank, service, inspection, education, and quality control. Performance assessment is one of the activities that can be carried out regularly throughout the maintenance and repair phase to determine the medical equipment’s actual condition.
Executing the assessment requires information concerning medical equipment features to produce the expected output. The expected output will assist healthcare management or clinical engineers in making essential decisions on maintenance management practises to enhance the reliability and availability of medical equipment. Furthermore, specific studies on assessment techniques within the South East Asia region, particularly in compliance with the Malaysian standard for managing medical equipment maintenance, are still lacking. In developing the present systematic review, the following research questions were addressed:
• What are the significant parameters required on the medical equipment to be applied for the reliability assessment from the previous studies?
• How do these parameters applicable to the Malaysian standard practises for managing the maintenance of medical equipment?
Selecting the significant parameters to be considered for medical equipment reliability assessment is very crucial in ensuring optimum healthcare services. In this study, the identification of these significant parameters can be applied for various types of medical equipment utilised in any healthcare institutions. In addition, we provide the review on feasibility of prediction of medical equipment reliability analysis using artificial intelligence (AI) and/or machine learning (ML) techniques based on these parameters throughout the maintenance phase of the medical equipment’s life cycle. This study also leads to the revealing of the gap and novelty. The identified parameters will contribute to the comprehensive and strategic maintenance management of medical equipment, which cover three main elements of preventive maintenance (PM), corrective maintenance (CM), and replacement plan (RP). Furthermore, the reliability assessment using these parameters may fulfil and improve the medical equipment maintenance’s national standard. Based on the study undertaken, none of included studies contributed on these three aspects and correlates the parameters with the relevant standards. Hence, the study aimed to identify the significant parameters of medical equipment by undertaking a systematic review of previous studies and correlate with the Malaysian standard of medical equipment maintenance management.
Materials and Methods
Literature Search
The systematic literature review was performed by applying the published standard, namely PRISMA in evaluating and rigorously analysing the articles related to medical equipment assessment in the databases (13). Besides, the inclusion and exclusion processes of the relevant current studies were thoroughly performed. The examination of the included study is coded to achieve the systematic review’s objective in the subject area.
Resources
The studies related to medical equipment assessment was from two primary databases, namely Web of Science and Scopus. The databases cover more than 256 studies field, including engineering and computer science studies that may increase the comprehensiveness and qualities of the article (14, 15). According to Younger (16), several established databases should be included to enhance the possibility of achieving the relevant articles in the subject area. In this study, the selected additional databases were PubMed, Science Direct, IEEE Xplore, Emerald, Springer, Medline, and Dimensions.
Article Selection
This stage elaborates the articles selection process. There are three steps in selecting the relevant articles, namely identification, screening, and eligibility.
Identification
The identification and selection of the relevant studies comprise four main stages. Firstly, the subject areas’ keywords were identified. The thesaurus, encyclopaedia and past researches were referred to construct appropriate keywords. Secondly, search string algorithms were developed from the keywords in January 2020 based on the Web of Science and Scopus databases’ characteristics, as illustrated in Table 1. Next, several inclusions and exclusion criteria were determined to retrieve the articles from both databases (Refer to Table 2). These criteria were set because only the latest research articles in the subject area were retrieved to minimise the possibility of irrelevant topic inclusion. Besides, only English articles were considered for easier analysis preparation. Subsequently, these two search strings were applied in the databases’ advanced search. Resultantly, 183 articles from Web of Science and 505 articles from Scopus were retrieved. Similar keywords were applied in the seven other databases, where 64 articles were identified. Moreover, identification of relevant studies was carried via other methods, which are websites, organisations, and citation searching (17). By using the similar keywords, there were 98 references were identified, Thus, 852 references consist of the articles and reports were retrieved in the identification stage.
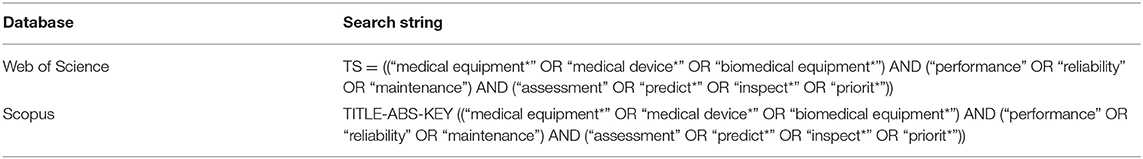
Table 1. The search strings for Web of Science and Scopus databases.
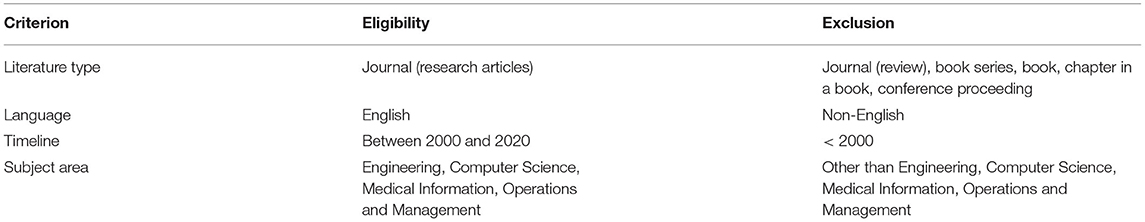
Table 2. The inclusion and exclusion criteria.
Screening
The 852 articles and reports were divided into two to remove duplication and exclude non-related subject areas or topics during the screening process. There were 38 and 19 repeated articles in the databases and other methods, respectively. Therefore, these duplicated articles were removed, and the remaining 716 articles and 79 reports progressed to a further screening process. Three features were properly examined during the screening process: the title, keywords, and abstract. Furthermore, several considerations were considered while examining the three features. Firstly, the general terms of medical equipment or medical device or other specific equipment categorised under these general terms were mentioned in the title and keywords. Secondly, an indication of the quantitative method in assessing the medical equipment performance was depicted in the abstract. Consequently, only 85 articles and 21 reports were selected to progress to the following step.
Eligibility
This step involved reviewing the articles’ full text to ensure that the 85 research articles and 21 reports were eligible to be synthesised and analysed. The articles’ significant contents were comprehensively scrutinised to confirm that the inclusion and exclusion criteria were fulfilled. Essential elements such as study aim, input parameters, methodology technique, expected output, and desired outcomes were thoroughly assessed. Subsequently, 69 articles and 21 reports were excluded due to not utilising the quantitative method to assess the medical equipment performance and not empirical studies. Besides, an additional two relevant articles were included based on hand-searching. Therefore, a total of 16 remaining articles were included in this study, as illustrated in Figure 1.
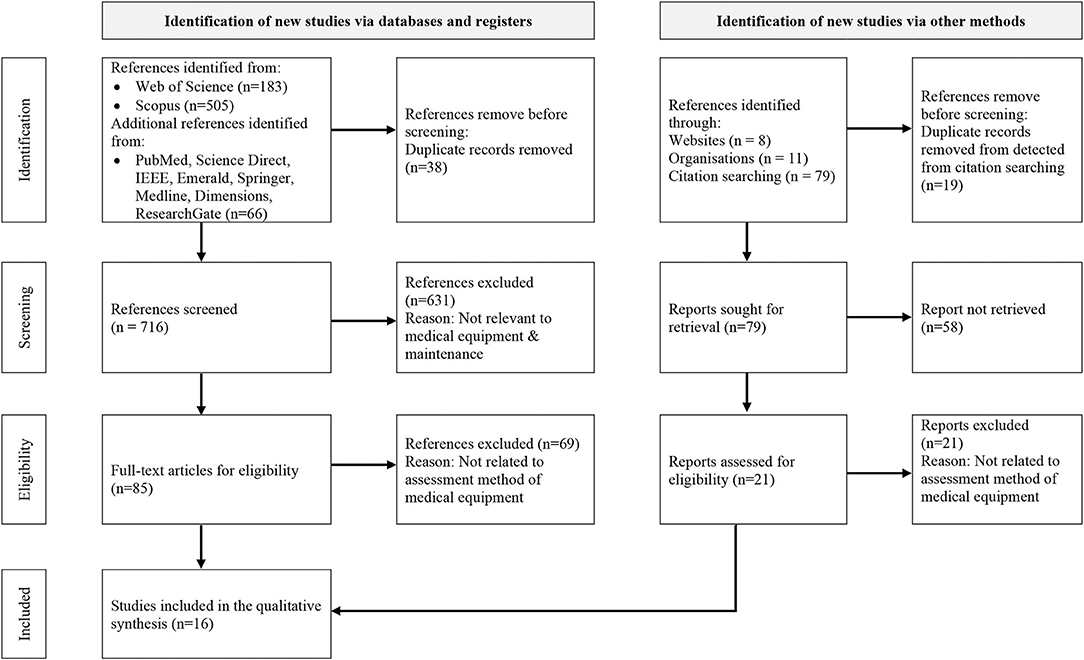
Figure 1. PRISMA flow chart of the study adapted from Page et al. (17).
Quality Assessment and Data Extraction
The qualitative analysis technique was used to assess the remaining articles. The first, second, and sixth authors performed the quality assessment of selected articles. The articles were categorised into high, moderate, and low levels that reflect the aim, input parameters, methodology technique, expected output, and desired outcomes (18). The articles must reach a high level and be agreed upon by all the authors. The compilation of extracted information was carried out by the first, second, and sixth authors and synthesised in an organised table. The third, fourth, and fifth authors subsequently checked all the synthesised data. The synthesised data was categorised by applying the thematic analysis. According to the Active Medical Device Maintenance Management developed by the Department of Standard, Malaysia, the established categories were correlated with the features. The result of input features categorisation was prudently discussed among authors. Any discrepancies or inconsistencies were resolved by consensus and until reaching reviewers agreement.
Results
Overall Background and Studies Findings
The analysis was carried out on 16 articles included in this study, as presented in Table 3. Based on this table, we concluded that none of the studies performed comprehensive analyses which include PM, CM, and RP. The selected studies included either one of the medical equipment reliability assessments of PM, CM, RP, and/or combination of either type of assessment. All articles were reviewed, and the motivations of each study were analysed and extracted. The articles’ common traits were identified, and potential research gaps were determined. Currently, no proper protocol and early intervention exercise in assessing the performance of medical equipment were reported. The healthcare organisation faces difficulty in implementing effective maintenance management of medical equipment without proper methodological procedure and planning. Thus, the medical equipment is unable to correctly operate and could be harmful to patients and users.
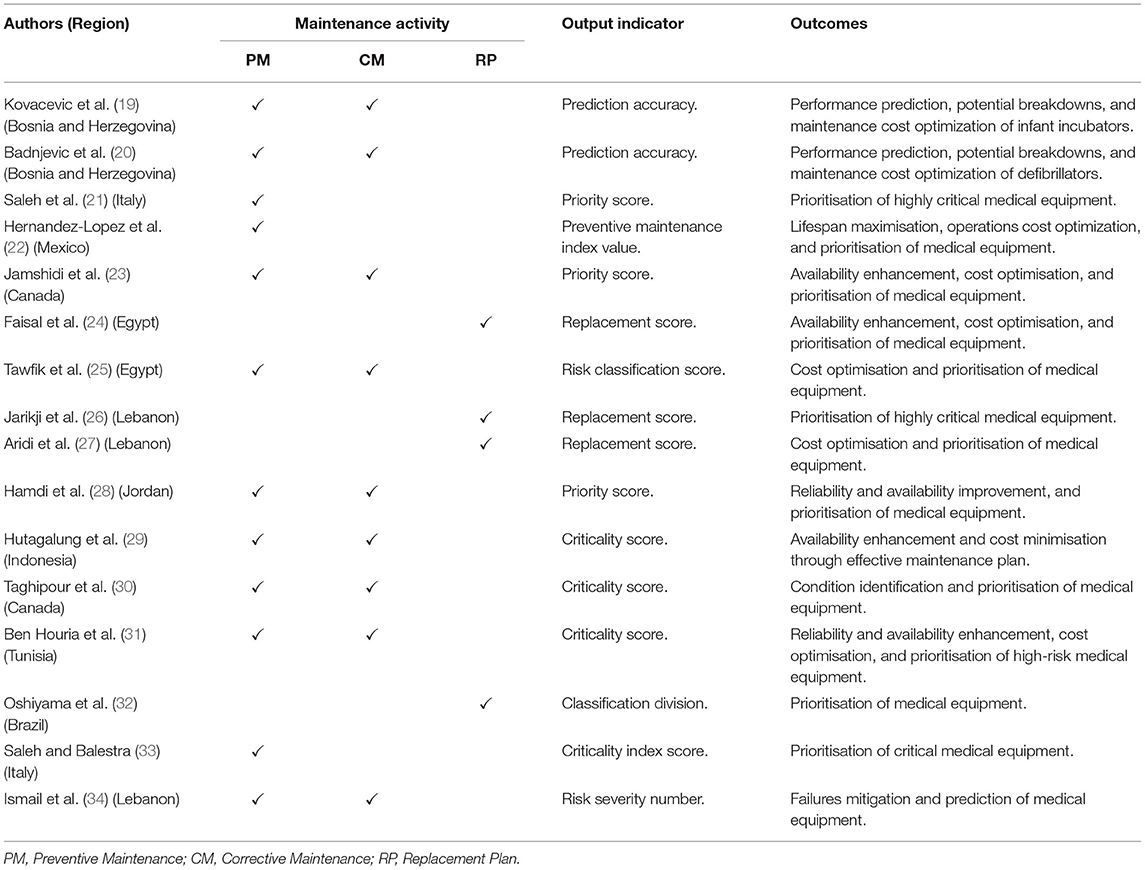
Table 3. Authors, maintenance activity, output indicator, and outcomes.
Since a large number of medical equipment and multiple functions are utilised in healthcare institutions, the equipment shall be monitored and correctly maintained to sustain performance and safety levels. However, maintenance management could be challenging if the healthcare provider encounters several problems regarding insufficient competent personnel and available resources, such as replacement parts and funds. According to the World Health Organisation (WHO), initial expenses, and operating expenditures are two categories of required financial resources in maintaining medical equipment (35). Corciova et al. (36) added that maintenance expenses represent a significant portion of the entire healthcare system which required 15–60% of the total cost to operate. Improper maintenance may affect performance and safety which greatly gave significant impact on the expenditure of healthcare institutions (37). Wu et al. (38) proved that practising effective maintenance management within 2 years improves the medical equipment availability and minimises the operating costs which exceeded one million dollars.
The computerised inventory system significantly assists healthcare management in managing equipment and maintenance activities. Applying the appropriate methodological technique in processing big data generates useful indicators that may assist clinical engineers in strategising the maintenance planning and further action course. The identification of the medical equipment criteria is essential to produce valuable indicators. Based on the analysis from the included articles, the justification for the criteria identification was referred from the previous literature, data collection and extraction, expert judgement via survey, input based on customer requirement, and adapting from international standards and national guidelines.
The relevant data were collected, processed, calculated, and analysed accordingly based on the identified criteria. Only one scientific methodological technique was involved in generating the expected output by referring to the 12 articles (19–30). Nevertheless, according to Ben Houria et al. (31), a combination of three techniques generated the expected output. The combination of two techniques was observed in the studies performed by Oshiyama et al. (32), Saleh and Balestra (33), and Ismail et al. (34), respectively. The proposed techniques were tested on the real dataset of various types of medical equipment particulars and maintenance information within a specific period.
The conclusion from the review of the 16 articles is that the healthcare institutions are capable of optimising the maintenance cost, improving the monitoring activity, managing the maintenance activities with available workforces and resources, prioritising PM and CM, assessing the equipment’s actual lifespan for the purpose RP, selecting the best maintenance management strategy based on the current situation by referring to the output indicator.
Main Findings
The outcomes from the selected articles using thematic analysis produced eight categories of significant parameters in assessing the medical equipment condition and reliability. The application of thematic analysis was carried out to develop the appropriate themes of medical equipment’s parameters. Based on the thematic analysis, these parameters are found to be significant for the AI/ML network as the input parameters. The initial stage of theme development procedures was the compilation of each parameter extracted from the 16 selected articles. In this phase, the categories of input parameters were properly analysed to extract the description used in the selected articles to address the research gaps. Then, in the second phase, various terms of input parameters were converted into general category via the themes, applications, or ideas classification. From the analysis, we found out that there were many terms used in previous studies, however, several of them can be addressed under the same group. Eventually, the thematic analysis has generated a total of eight categories of input parameters, which described the characteristics of medical equipment utilised in healthcare institutions.
The observation was also carried out according to the studies outcomes. There were seven categories of input parameters extracted from the selected articles as tabulated in Table 4. In general, the outcome was to classify the medical equipment in accordance with maintenance management activities. The medical equipment maintenance activities comprise of PM, CM, and RP. The prioritisation was made based on the medical equipment characteristics toward the strategic maintenance management activity.
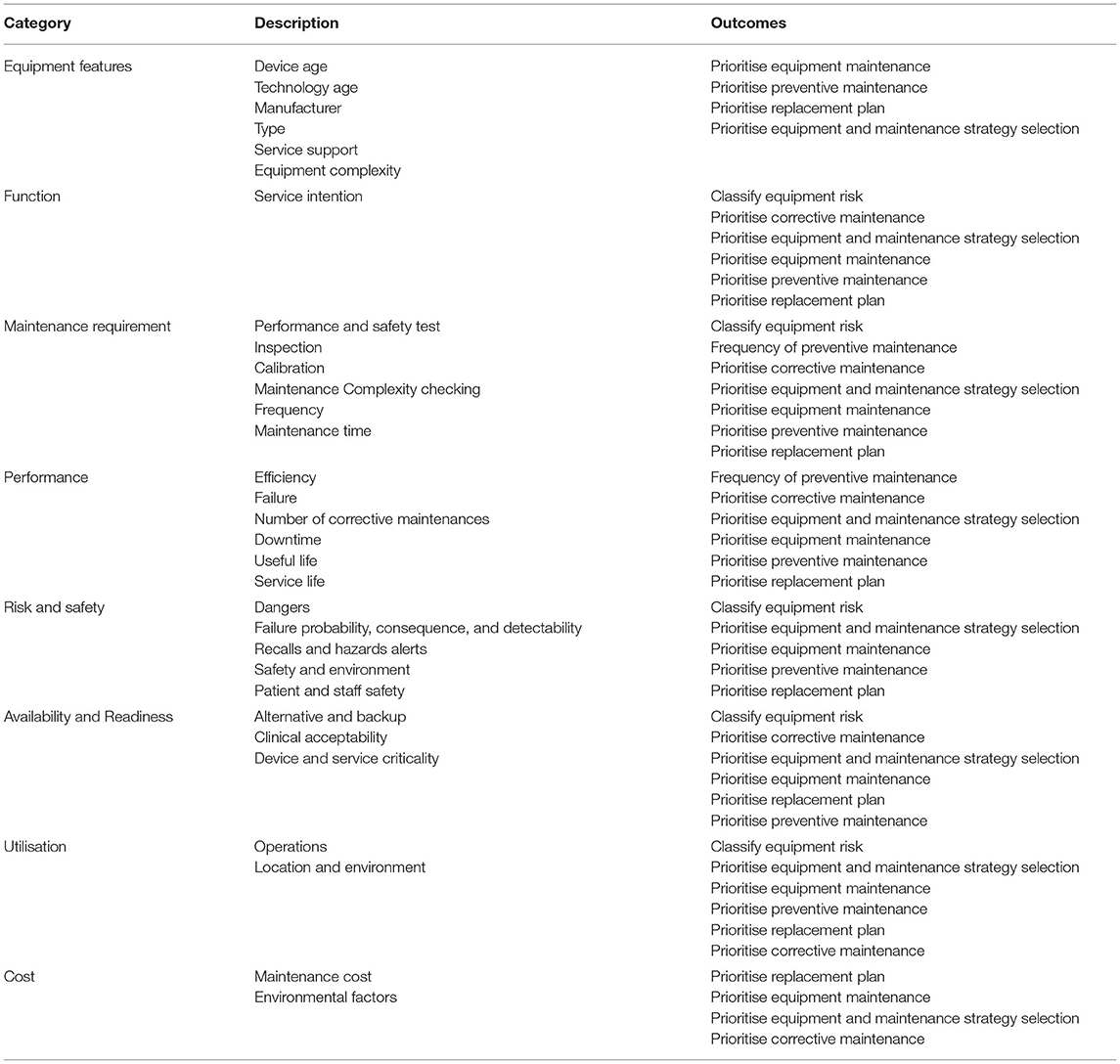
Table 4. Categories of input parameters.
The generated eight categories of input parameters will assist the clinical engineers to perform the medical equipment reliability assessment. Understanding of the equipment reliability may lead to proper maintenance activity, which subsequently increase the availability of medical equipment with optimised resources. The measurement of these parameters using AI/ML techniques will comprehensively enhance the monitoring of medical equipment performance and utilisation status through predictive maintenance model. This predictive model is able to mitigate the potential failures, deterioration, and obsolescence. Findings from the selected articles produced eight categories of the significant input parameters in assessing the medical equipment condition, as tabulated in Table 4.
Equipment Features
Equipment features comprise several characteristics designed for the equipment and exist since the unit is manufactured. One of the parameters used to assess the medical equipment condition is age (19, 20, 22–24, 26, 27, 29–31). The equipment age reflects the overall condition of the equipment. This is because, the equipment is typically performed well at an early age, and fewer failures are observed. However, as the age increases, the equipment starts to degrade. In another studies conducted by Badnjevic et al. (20) and Kovacevic et al. (19), manufacturer’s name and equipment’s modality are another two parameters that were included in their reliability assessment. Prior to that, Faisal et al. (24), included the service availability as one of the input parameters in assessing the equipment condition for the RP prioritisation. The service availability of the medical equipment includes warranty, documentation, training, and compatible spare part. Meanwhile, studies by Saleh et al. (21) and Saleh and Balestra (33) also considered equipment complexity as the input parameter in their medical device reliability assessment.
Function
The function of the equipment here reflects on the intended use of the equipment in healthcare services delivery. The equipment function is divided into several forms of services namely life support, therapeutic, diagnostic, analytical, and miscellaneous.
Maintenance Requirement
Maintenance requirements involve activities and tasks to ensure that the medical equipment is sustained in the expected condition in terms of functionality and physical. The complexity of carrying out the maintenance procedure is different among the equipment types. The maintenance, servicing, or restoration of this equipment requires a skilled person to dismantle and replace the replacement or faulty part. The procedure is also time-consuming. Thus, the equipment can be unavailable for an extended period if the performance of maintenance activity is carried out ineffectively.
Performance
The reliability of the medical equipment highly depended on the performance that can be measured from the efficiency and uptime. The equipment effectiveness can be observed from the usage and service life. The performance of medical equipment should be vitally monitored and ensured as described by the manufacturer. The excellent performance of medical equipment can mitigate the interruption of healthcare services to the public.
Risk and Safety
In delivering the healthcare services such as diagnosis and treatment, patients and clinicians must be kept safe without exposure to any hazard that may cause severe injury. The risk and safety of the equipment can be predicted by studying the failure aspects (23, 28, 30). The authority may issue the recalls and hazards alert if any incident occurs involving medical equipment utilisation by instructing the user to immediately stop using the equipment to prevent further possible danger to clinicians and patients (23, 30). In addition, hazards may when mishandling, misdiagnosis, inappropriate treatment or error are made by the operator (30, 31). Hazards may also exist from the operation or physical of the medical equipment (21, 25, 33).
Availability and Readiness
The availability and readiness of medical equipment are vital to ensure that healthcare services to patients can be delivered without compromise. The category consists of the alternatives and backup units, device criticality, and user acceptability. The breakdown of equipment is inevitable due to normal wear and tear, or ageing may interfere with the effectiveness of healthcare services. Moreover, the importance of healthcare services will cause equipment to become a critical necessity due to unavoidable circumstances (25–27, 29–31). Thus, alternative units must be ready for critical times (24, 25, 27–31).
Location
The assessment of the medical equipment condition can also be undertaken by observing the unit utilisation level. The utilisation level can be affected by the location of equipment and the situation where the equipment is used to deliver healthcare services to the public (21, 22, 26, 33). According to the authors, the frequency of medical equipment usage and turning to be essential depending on the type and activity of healthcare services provided to patients, such as anaesthetising, operating theatres, and others. Furthermore, the equipment may be extensively or rarely used depending on the healthcare services situation.
Cost
Cost is one of the crucial aspects in managing medical equipment maintenance and replacement activities. According to the studies performed by Ben Houria et al., reducing the cost of operations involving medical equipment is crucial. The operational costs must be below the allocated budget (31). Findings from Faisal et al. (24) reported that maintenance costs should not be over 25% of the procured medical equipment cost over the past 3 years. In other study conducted by Oshiyama et al. (32) suggested that the CM cost should be within 3 to 5% of the medical equipment purchased price. According to Hutagalung et al. (29), maintenance costs can be reduced by enhancing the availability of medical equipment through effective maintenance management. With regards to the previous findings stated before, the maintenance cost highly reflected on the reliability of the medical equipment. The frequent failures do not only lead to the excessive maintenance tasks and disruption to the healthcare services, but also involve extra expenses. When the equipment requires high maintenance operation and the imposed cost reached to the specific limits, the equipment is no longer reliable and fit for utilisation. This condition is known as beyond economic repair.
Discussion
Overall Findings
Previous studies demonstrated the importance of assessing medical equipment in planning for necessary action within healthcare institutions. The first important consideration in developing the medical equipment performance assessment is determining the appropriate input parameters (12). However, no single technique can be applied to all the input parameters. The selection of input parameters must be appropriate and applicable to the expected output. According to Mahfoud et al. (39), the outcome of the medical equipment assessment associates with the maintenance strategies. The availability of an existing dataset comprising medical equipment details and maintenance history is one of the factors in selecting the appropriate input parameters. The difference in input parameters applied can be processed to generate similar output.
The second consideration is the optimum processing technique based on the myriad of medical equipment data. As mentioned earlier, many scientific methods were developed which can be used to compute the input data and eventually generate an expected output for assessment purposes (40). However, the ML technique application is observed to be a better technique compared to the conventional techniques. This is due to the capability of the ML algorithm in testing the predictive high output accuracy by applying the accurate and significant input data.
The results obtained from the studies made by Badnjevic et al. (20) and Kovacevic et al. (19) showed that the generated output achieved above 89% accuracy where Random Forest and Decision Tree reached around 99% of accuracy in predicting both selected medical equipment performance. Therefore, both authors concluded that improved supervision, quality and safety in managing medical equipment maintenance could be achieved which eventually optimised the cost of maintenance. However, the ML techniques utilised in both studies were developed based on only one type of medical equipment. Consideration of applying to various types of medical equipment would be more practical to be utilised in healthcare facility management. This is because, various types of medical equipment have difference functionality and required specific assessment to ensure their reliability to be used in healthcare services.
The third consideration in developing the medical equipment condition assessment technique is to determine the expected output. One of the indications in identifying the expected output is observing the trend of the occurred problems (41). From the list of observations, the trend is translated into a specific objective to resolve the problem. The review concludes that the clinical engineers faced several common issues, such as the unavailability of medical equipment due to malfunctioning, insufficient workforces (i.e., competent technical staff), and limited resources (i.e., limited resources budget). Effective maintenance management must be established to overcome these problems and prevent severe consequences. The prioritisation by assessing the existing medical equipment condition can be undertaken while working within the current workforce and resources.
The Medical Equipment Reliability Assessment From the Malaysian Perspective
The Malaysian government spent approximately RM27 million in 2018 for new procurement and upgrading initiatives of medical equipment in public healthcare facilities to provide efficient healthcare services to the public (42). The Malaysian government also executed a new leasing programme involving six main medical equipment for 5 years starting from 2019, comprising a maintenance scheme with an approximate cost of RM19.7 million.
In the private sector, KPJ Healthcare Berhad, a leading private healthcare service in Malaysia with more than 20 hospitals throughout the country, procured medical equipment worth around RM136 million in 2019, showing an increment of 32% from the previous year (43). The evidence from both sectors indicates that massive investment in procurement and maintenance of medical equipment is necessary in delivering effective healthcare services to the community. Therefore, the efficient maintenance management of medical equipment during operations is vital to maximise the life span of the unit and ensure the investment is worthy.
During the eleventh Malaysia Plan, there was a need of highly technological medical equipment to meet the essentials for various kind of diseases advanced management (44). These critical machines such as computerised tomography (CT) and magnetic resonance imaging (MRI) were required in facilitating the medical practitioners for detecting, diagnosing, and treating the critical diseases. The National Medical Devices Survey was conducted in both public and private healthcare sectors and found out that the ratio of MRI number and population was two per million, whereas CT was 4 per million. This finding indicated the lower ratios than of Organisation for Economic Co-operation and Development (OECD) countries.
Referring to the Ministry of Health report for three consecutive years starting from 2017, the percentages of hospitals and public health facilities outpatient attendees had slightly increased in average of 2.64%, whereas the hospitals day care attendees had significantly escalated to 15.8% (45–47). Furthermore, the hospitals admissions had marginally increased to 5.96%. This indication drove the Malaysian government to expand the secondary and tertiary care services. From the year of 2015–2018, the number of hospital beds was increased by 3.3%, in which the increment of 11% applied to intensive care units (48). Although the improvement was made, the existing ratio of 1.9 beds to every 1,000 Malaysia populations is still below than the initial target. From the initiative programme implementation, the Malaysian government provides 67% of total beds in the country. Other expansion programmes that initiated by the Malaysian government were the development a new replacement hospital in east-coast region, the extension of the new complexes in the existing hospitals, and the new hospital in central region. These development activities require the expansion of facility, equipment, manpower, and services.
Apart from this expansion initiative, the medical-based day care services such as paediatric, oncology, and haematology require specific medical equipment for chemotherapy, blood transfusion, and haemodialysis (49). Therefore, the Ministry of Health Malaysia has come out with a general policy to enhance the availability of medical equipment for strengthen the healthcare services in the country. Besides, based on the inspection carried out by the internal audit of Ministry of Health Malaysia has recommended that the improvement of medical equipment is required to facilitate the healthcare services to the public (50).
The application of medical equipment in facilitating the healthcare services is crucial. The Malaysian government provided a huge amount of funds in procuring and maintaining the medical equipment in the country. The procurement of medical equipment includes for a new development of hospitals and replacement of the disposed units due to beyond economic repair (42).
Subsequently, this sub-section discusses the correlation on eight categories of input parameters as set by the Malaysian standard, namely the Code of Practise for Good Engineering Maintenance Management of Active Medical Devices (MS 2058:2018) (51). The primary function of this body is to encourage and promote global competitiveness through reliable standardisation and accreditation services governed by the Standards of Malaysia Act 1996 Act 549 (52). Several normative references comprising other interconnected MS 2058:2018 guidelines and national acts are available to develop a comprehensive standard and comply with the legislative requirement.
One crucial related act, specifically for managing the medical equipment, is the Medical Device Act 2012 (53). This act is intended to provide statutory regulation for medical devices in Malaysia and shall be complied with by all relevant parties, such as authorised representatives, manufacturers, and service providers. This act is also under the enforcement of the Malaysian governmental regulatory body, namely the Medical Device Authority, based on the establishment of the Medical Device Authority Act 2012 (54). Act 737 regulates the entire life cycle of medical equipment covering three main phases, namely pre-market, placing in market and post-market.
According to Annex P of MS 2058:2018, ten factors are proposed to assess the medical equipment for the RP. Based on the observation and comparison made with the included studies as shown in Table 5, these factors can also be used as an input to assess the medical equipment condition for maintenance prioritisation.
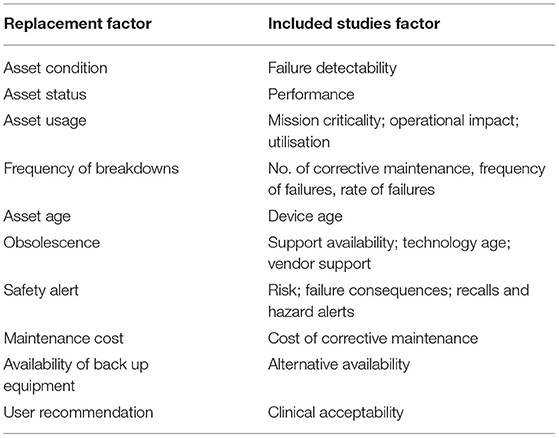
Table 5. Comparison between factors proposed in MS 2058:2018 and included studies.
Firstly, according to the observation of the factors proposed in the MS 2058:2018, the asset age is directly similar to the first category grouped based on the analysis in this study, namely the equipment features. Furthermore, most authors highly utilise equipment age to obtain an indication of prioritising maintenance and RP. The second factor that is similar to the equipment feature category was obsolescence. This factor is quite identical to service support because if there is no service support in the market, the restoration work involves replacement parts or any maintenance services for related equipment can be delivered. Therefore, the factors of asset age and obsolescence are similar to equipment features.
The results of comparisons between all the factors proposed in the MS 2058:2018 with two of the eight categories, namely function and maintenance requirement, found no similarity. Next, the performance category comprises several parameters involving efficiency, failure, downtime, uptime, and the number of corrective maintenances performed. In comparison with the MS 2058:2018, the performance category seems equivalent to the frequency of breakdown where the medical equipment performance can be measured by assessing the failure rate. Furthermore, factors such as asset status and asset condition seem to be related to this category.
The next category initiated in this study was risk and safety. This category is crucial to mitigate any potential hazards posed to the patient and clinician. From the comparison made, the factor of safety alert proposed in MS 2058:2018 correlated with the risk and safety category. The correlation was due to the risk and safety category involving the recalls and hazards alerts that can be declared or issued by the local authority body (53), manufacturer or locally authorised representative. The availability and readiness were in a category consisting of the element of correlation between equipment and service criticality. In MS 2058:2018, the factors that were observed as most similar were the availability of backup equipment and user recommendation. These factors demonstrate the criticality in ensuring equipment availability in assisting the healthcare services at the clinically acceptable level.
Asset usage is one of the 10 factors proposed in MS 2058:2018 that indicates the extensive utilisation level of the medical equipment. Direct similarity with the category of utilisation was apparent when compared against the categories in this study. A direct similarity can be identified between the maintenance cost factor proposed in MS 2058:2018 and the cost categorised in this study. Table 6 tabulates the correlation between the eight categories summarised based on the review in this study with the 10 factors proposed in MS 2058:2018.
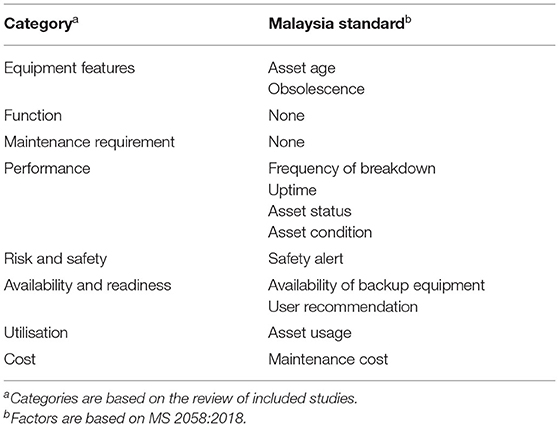
Table 6. Correlation between study categories and factors in MS2058:2018.
The national standard of MS 2058:2018 helps the clinical engineers significantly in managing the maintenance of medical equipment in Malaysia. There are several vital relevant acts and standard were referred for the development and compilation of this national standard. It covers various types of equipment in all maintenance stages starting from equipment acceptance to disposal process. This standard also includes main and sub-element of maintenance activities, which are PM, CM, and RP. The ultimate purpose is to ensure a proper maintenance is performed for continuous performance of medical equipment.
The adherence of MS 2058:2018 may optimise the performance of medical equipment utilised in healthcare institution. The optimised performance of medical equipment reduces error while delivering the healthcare services to public. Any difference in measurement could give bad impact on the healthcare services especially to the patients. It is important to ensure that the equipment can give accurate and precise measurement during therapeutic, diagnostic, and analytic procedures. The reliability of healthcare services really depends on effectiveness of medical equipment. The optimised equipment does not only assist during healthcare procedures, but improve the availability of the equipment while needed. Moreover, it also upkeeps in terms of the safety aspect from any possible failure, which may cause severe harmful against the users and patients. Thus, the optimization of medical equipment performance may upkeep the level of healthcare services and reduce the cost of operations.
Conclusion
The current study on the medical equipment assessment indicated a fundamental understanding of how the assessment contributes toward the effectiveness in delivering healthcare services to the community. The ultimate contribution from this study is that the healthcare institutions are capable of providing better healthcare services to the public by the medical equipment availability, upkeep the safety level by avoiding any failures of equipment that may cause hazards, and prepare and allocate sufficient budget on the expenditure of equipment maintenance and replacement activities.
The review summarised that the assessment of medical equipment conditions will assist the clinical engineers to increase the availability of the equipment in healthcare institutions. Furthermore, the equipment availability will assist the clinical engineering department in healthcare institutions to achieve medical equipment maintenance management effectiveness by prioritising the activity according to the urgency. This prioritising approach may eventually optimise the operational cost and work with available resources.
According to the comparison of the findings in this study with MS2058:2018, the categories classified based on the input parameters extracted from the previous studies and have been correlated with proposed factors in the national standard. However, improvement can be made by adding several factors that covered the function and maintenance requirement categories. The proposal for selecting the input parameters depends on clinical engineers’ required objective to overcome the current issue experienced in healthcare institutions. The selected parameters can be practical with the support of accurate existing data.
The expected beneficial output indicated from the assessment technique depends on the experimental input parameters. The output can be generated through systematic processes and professional ways rather than the perception by adapting the appropriate methodological technique. This output can assist clinical engineers in making a proper decision to take the right action to overcome the current issue. Therefore, this study provides recommendations that will be useful for future research:
1) Development of a comprehensive strategic medical equipment maintenance management that covers three main activities, which are PM, CM, and RP. The system shall prioritise the medical equipment at each maintenance activity by measuring the criterion of input parameters proposed in this study. In addition, considering these three activities will provide a comprehensive assessment to the healthcare providers in prioritising resources and proposing appropriate solution in timely manner.
2) Applying ML techniques in assessing the medical equipment condition and reliability. The predictive nature of ML will provide active action in healthcare industry in anticipating medical equipment’s failures. Current solutions are based on passive actions which greatly impacted healthcare services providers. Thus, the advancements of ML techniques are deemed to be a practical solution in medical equipment predictive maintenance in mitigating severe failures, optimising resources, improving availability, and upkeeping performance.
3) Imposing adaptive framework on medical equipment reliability based on the functionality of healthcare providers. The capability of ML algorithms in predicting prioritisation of medical equipment maintenance will enable accurate and precise assessment based on the contributing factors of the medical equipment. The framework will be adaptive to the nature of the healthcare institutions’ function since the predictive nature of ML algorithm able to find patterns and trends based on the previous scenarios. Thus, specific model can be easily adopted by healthcare providers in ensuring optimised services to community.
Therefore, by applying appropriate techniques that drive the compliance of national standards and statutory requirements, the review provided a new insight in adopting AI and/or ML algorithms which can be aligned in any government’s standard in providing better healthcare services.
Author Contributions
AZ and KH designed and developed the study protocol as well as major contributors to the article writing. AZ, AA, and KH performed the identification, screening, eligibility, quality assessment, and information extraction of the articles. MA, SS, and KL checked all the synthesised data and approved the final version to be submitted for publication. All authors have substantial contributed to the article.
Funding
This work was supported by the University Malaya Research Grant Faculty Programme (RF010-2018A) and International Funding from Motorola Solution Foundation (IF014-2019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express thanks to the Malaysian Ministry of Health for the continuous supports in conducting this study.
Abbreviations
AI, Artificial Intelligence; CM, Corrective Maintenance; ML, Machine Learning; MS2058:2018, Code of Practise for Good Engineering Maintenance Management of Active Medical Devices; PM, Preventive Maintenance; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses; RP, Replacement Programme; WHO, World Health Organisation.
References
1. Chaudhary P, Kaul P. Factors affecting utilization of medical diagnostic equipment: a study at a tertiary healthcare setup of Chandigarh. CHRISMED J Health Res. (2015) 2:316. doi: 10.4103/2348-3334.165741
CrossRef Full Text | Google Scholar
3. Yik Francis WH, Lai Joseph HK, Yuen PL. Impacts of facility service procurement methods on perceived performance of hospital engineering services. Facilities. (2012) 30:56–77. doi: 10.1108/02632771211194275
CrossRef Full Text | Google Scholar
4. Shohet Igal M, Lavy S. Healthcare facilities management: state of the art review. Facilities. (2004) 22:210–20. doi: 10.1108/02632770410547570
CrossRef Full Text | Google Scholar
5. Cesarotti V, Di Silvio B. Quality management standards for facility services in the Italian health care sector. Int J Health Care Qual Assur. (2006) 19:451–62. doi: 10.1108/09526860610687600
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Wang B. Medical equipment maintenance: management and oversight. Synthesis Lectures Biomed Eng. (2012) 7:1–85. doi: 10.2200/S00450ED1V01Y201209BME045
CrossRef Full Text | Google Scholar
8. World Health Organization. Development of Medical Device Policies: World Health Organization, Geneva (2011). 39 p.
Google Scholar
9. Worm A. Managing The Lifecycle of Medical Equipment. THET (2015).
10. Salim S, Mazlan S, Salim S. A conceptual framework to determine medical equipment maintenance in hospital using RCM method. MATEC Web Conferences. London (2019) 266:02011. doi: 10.1051/matecconf/201926602011
CrossRef Full Text | Google Scholar
11. Kutor J, Agede P, Ali R. Maintenance practice, causes of failure and risk assessment of diagnostic medical equipment. J Biomed Eng Med Devices. (2017) 02:123. doi: 10.4172/2475-7586.1000123
CrossRef Full Text | Google Scholar
12. Bahreini R, Doshmangir L, Imani A. Factors affecting medical equipment maintenance management: a systematic review. J Clin Diagnostic Res. (2018) 12:IC1–7. doi: 10.7860/JCDR/2018/31646.11375
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Martin-Martin A, Orduna-Malea E, Thelwall M, Lopez-Cozar ED. Google scholar, web of science, and scopus: a systematic comparison of citations in 252 subject categories. J Informetr. (2018) 12:1160–77. doi: 10.1016/j.joi.2018.09.002
CrossRef Full Text | Google Scholar
15. Adriaanse LS, Rensleigh C. Web of science, scopus and google scholar a content comprehensiveness comparison. Electron Libr. (2013) 31:727–44. doi: 10.1108/EL-12-2011-0174
CrossRef Full Text | Google Scholar
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Informat. (2018) 34:285–91. doi: 10.3233/EFI-180221
CrossRef Full Text | Google Scholar
19. Kovacevic Z, Pokvic LG, Spahic L, Badnjevic A. Prediction of medical device performance using machine learning techniques: infant incubator case study. Health Technol. (2019) 10, 151–5. doi: 10.1007/s12553-019-00386-5
CrossRef Full Text | Google Scholar
20. Badnjević A, Gurbeta Pokvić L, Hasičić M, Bandić L, Mašetić Z, Kovačević Ž, et al. Evidence-based clinical engineering: machine learning algorithms for prediction of defibrillator performance. Biomed Signal Process Control. (2019) 54:101629. doi: 10.1016/j.bspc.2019.101629
CrossRef Full Text | Google Scholar
21. Saleh N, Sharawi AA, Abd Elwahed M, Petti A, Puppato D, Balestra G. Preventive maintenance prioritization index of medical equipment using quality function deployment. IEEE J Biomed Health Informat. (2015) 19:1029–35. doi: 10.1109/JBHI.2014.2337895
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Hernández-López LA, Pimentel-Aguilar AB, Ortiz-Posadas MR. An index to prioritize the preventive maintenance of medical equipment. Health Technol. (2019) 10:399–403. doi: 10.1007/s12553-019-00371-y
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Jamshidi A, Rahimi SA, Ait-kadi D, Ruiz A. A comprehensive fuzzy risk-based maintenance framework for prioritization of medical devices. Appl Soft Comput. (2015) 32:322–34. doi: 10.1016/j.asoc.2015.03.054
CrossRef Full Text | Google Scholar
24. Faisal M, Sharawi A. Prioritize medical equipment replacement using analytical hierarchy process. IOSR J Electric Electron Eng. (2015) 10:55–63. doi: 10.9790/1676-10325563
CrossRef Full Text | Google Scholar
25. Tawfik B, Ouda BK, Abd El Samad YM. A fuzzy logic model for medical equipment risk classification. J Clin Eng. (2013) 38:185–90. doi: 10.1097/JCE.0b013e3182a90445
CrossRef Full Text | Google Scholar
26. Jarikji Y, Hussein B., Hajj-Hassan M. A quantitative model for replacement of medical equipment based on technical and economic factors. Int J Artificial Organs. (2019) 42:278–85. doi: 10.1007/978-3-030-30874-2_22
CrossRef Full Text | Google Scholar
28. Hamdi N, Oweis R, Abu Zraiq H, Abu Sammour D. An intelligent healthcare management system: a new approach in work-order prioritization for medical equipment maintenance requests. J Med Syst. (2012) 36:557–67. doi: 10.1007/s10916-010-9501-4
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Hutagalung AO, Hasibuan S. Determining the priority of medical equipment maintenance with analytical hierarchy process. Int J Online Biomed Eng. (2019) 15:107–20. doi: 10.3991/ijoe.v15i10.10920
CrossRef Full Text | Google Scholar
30. Taghipour S, Banjevic D, Jardine AKS. Prioritization of medical equipment for maintenance decisions. J Operat Res Soc. (2011) 62:1666–87. doi: 10.1057/jors.2010.106
CrossRef Full Text | Google Scholar
31. Ben Houria Z, Masmoudi M, Al Hanbali A, Khatrouch I, Masmoudi F. Quantitative techniques for medical equipment maintenance management. Europ J Indust Eng. (2016) 10:703–23. doi: 10.1504/EJIE.2016.081017
CrossRef Full Text | Google Scholar
32. Oshiyama NF, Bassani RA, D’Ottaviano IML, Bassani JWM. Medical equipment classification: method and decision-making support based on paraconsistent annotated logic. Med Biol Eng Comput. (2012) 50:395–402. doi: 10.1007/s11517-012-0888-6
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Saleh N, Balestra G. Comprehensive framework for preventive maintenance priority of medical equipment. Annu Int Conf IEEE Eng Med Biol Soc. (2015) 2015:1227–30. doi: 10.1109/EMBC.2015.7318588
PubMed Abstract | CrossRef Full Text | Google Scholar
35. World Health Organization. Medical Equipment Maintenance Programme Overview: WHO Medical Device Technical Series. Geneva: Department of Essential Health Technologies, World Health Organization (2011).
36. Corciovă C, Andritoi D, Luca C. A modern approach for maintenance prioritization of medical equipment. Maintenance Manage IntechOpen. (2020) doi: 10.5772/intechopen.92706
CrossRef Full Text | Google Scholar
37. Bahreini R, Doshmangir L, Imani A. Influential factors on medical equipment maintenance management: in search of a framework. J Qual Maintenance Eng. (2019) 25:128–43. doi: 10.1108/JQME-11-2017-0082
CrossRef Full Text | Google Scholar
38. Wu H, Liu G (editors). Building the quality control system for medical equipments in hospital. In: 2010 International Conference on Management and Service Science. London (2010). doi: 10.1109/ICMSS.2010.5576119
CrossRef Full Text | Google Scholar
39. Mahfoud H, El Barkany A, El Biyaali A. Medical maintenance performance monitoring: a roadmap to efficient improvement. Int J Productiv Qual Manage. (2017) 22:117–40. doi: 10.1504/IJPQM.2017.085850
CrossRef Full Text | Google Scholar
40. Jamshidi A, Rahimi SA, Ait-Kadi D, Bartolome AR (editors.). Medical devices inspection and maintenance; a literature review. In: Proceedings of the 2014 Industrial and Systems Engineering Research Conference. Wuhan: Institute of Industrial Engineers. (2014).
Google Scholar
41. Diani CA, Rock A, Moll P. An evaluation of the effectiveness of a risk-based monitoring approach implemented with clinical trials involving implantable cardiac medical devices. Clin Trials. (2017) 14:575–83. doi: 10.1177/1740774517723589
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Kementerian Kesihatan Malaysia. Laporan Tahunan Kementerian Kesihatan Malaysia 2018. In: Centre HI, editor. Malaysia: Kementerian Kesihatan Malaysia (2018).
Google Scholar
43. KPJ Healthcare Berhad. Financial Report 2019, Montreal (2019).
Google Scholar
44. World Health Organization. Malaysia Health System Review. Health Systems in Transition. Manila: WHO Regional Office for the Western Pacific (2012).
Google Scholar
45. Ministry of Health Malaysia. Health Facts 2018 (Reference Data for 2017). In: Planning Division HIC, editor. Malaysia: Ministry of Health Malaysia (2018).
Google Scholar
46. Ministry of Health Malaysia. Health Facts 2019 (Reference Data for 2018). In: Planning Division HIC, editor. Malaysia: Ministry of Health Malaysia (2019).
Google Scholar
47. Ministry of Health Malaysia. Health Facts 2020 (Reference Data for year 2019). In: Planning Division HIC, editor. Malaysia: Ministry of Health Malaysia (2020).
Google Scholar
48. Ministry of Health Malaysia. Strategic Framework of the Medical Programme, Ministry of Health Malaysia (2021 – 2025). Hospital Management Unit MDD, editor. Malaysia: Ministry of Health Malaysia (2020).
49. Kementerian Kesihatan Malaysia. Polisi Perkhidmatan Rawatan Harian di Hospital-Hospital Kementerian Kesihatan Malaysia. Perubatan BP, editor. Malaysia: Kementerian Kesihatan Malaysia (2016).
50. Ministry of Health Malaysia. Laporan Audit Dalam Tahun 2020. Malaysia: Ministry of Health Malaysia (2020).
51. Department of Standards Malaysia. Code of Practice for Good Engineering Maintenance Management of Active Medical Devices (Second revision). In: Standard M, editor. Kuala Lumpur: MS 2058:2018: Department of Standards Malaysia (2018).
52. Department of Standards Malaysia. Standards of Malaysia Act 1996 (Act 549). Cyberjaya: Department of Standards Malaysia (2012). p. 1–35. Available online at:
link







